In this reaction 1 mole of propane forms 4 moles of H₂O 1 mole of propane 4 moles of water. C H 2.
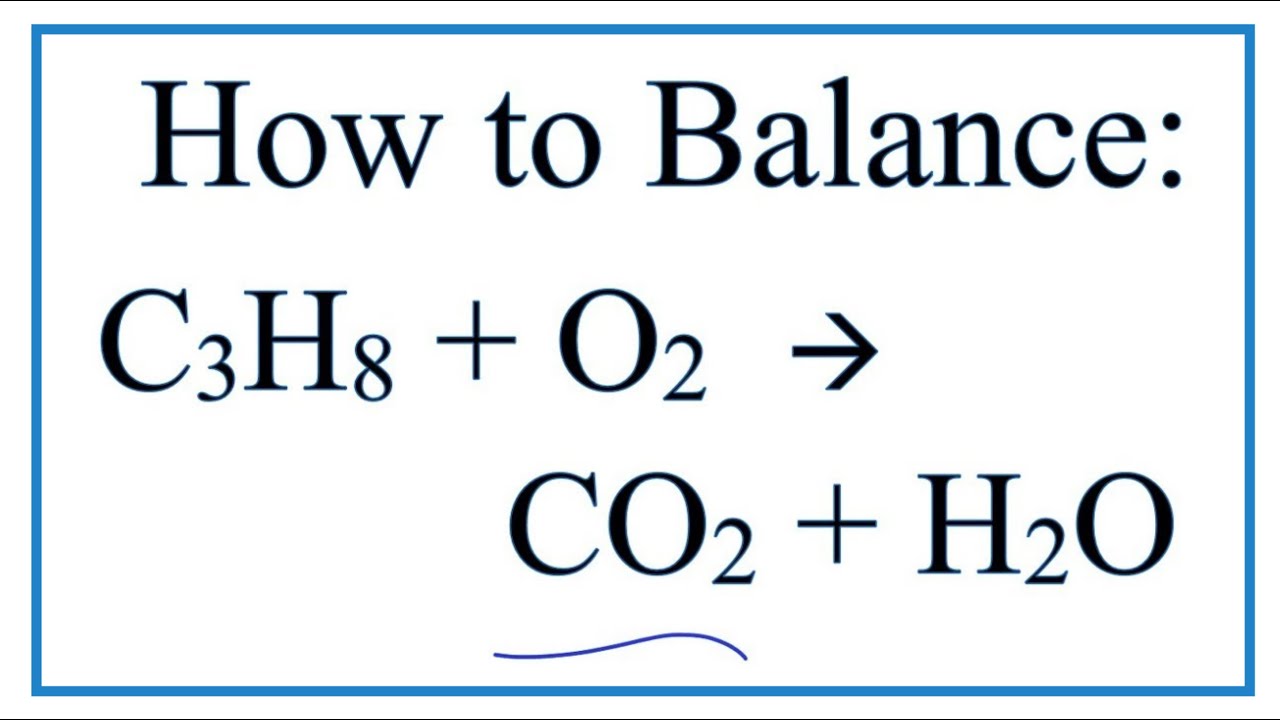
How To Balance C3h8 O2 Co2 H2o Propane Combustion Reaction Youtube
Assuming no hydrocarbons in the products the volume percentage of CO in the products is _______.

. How much heat energy. When C 3 H 8 is burned in oxygen the products are. Solving for X X 10 mol.
CO 2 H 2 O. If 2372 g of oxygen are consumed how many liters of. C3H8l 5 O2g - 3 CO2g 4 H2Og.
C_3H_8 g 5 O_2g 3 CO_2 g 4 H_2O g 30 mol CO_2 60 mol CO_2 20 mol CO_2 80. Write an unbalanced formula equation for the reaction. D CO2 H2O.
If 20 mol of propane are burned reacted with oxygen how many moles of carbon dioxide will be produced. See the answer See the answer done loading. When propane gas C3H8 is burned with oxygen the products are carbon dioxide and water.
C CO2 H2. When propane burns it reacts with oxygen O2. When C 3 H 8 is burned in oxygen the products are.
Exchange oxygen in the blood for carbon dioxide carry oxygenated blood away from the heart hold receptors for the sense of. You have to properly balance the equation before you can answer this question. Write an unbalanced formula equation for the reaction.
CH 2 H 2 O. When 0105 mol propane C3H8 is burned in an excess of oxygen how many moles of oxygen are consumed. The products are carbon dioxide and water.
Burns completely in oxygen which of the following products are formed. CO 2 H 2. A 0420 mol O2.
5when propane gas c3h8 is burned with oxygen the products are carbon dioxide and waterwrite an unbalanced formula equation for the reaction6. Heat A III and III B I and II only C II and III only D I only Easy Solution Verified by Toppr Correct option is A The reaction of burning of Ethane in air is given by the following equation- 2C 2 H 6 7O 2 4CO 2 6H 2 O Heat. Chemical reaction representing the burning of propane.
Solution for When propane is burned the balanced reaction is. Assuming that all volume measurements occur at the same. E 0975 mol O2.
Propane C3H8 is a fuel commonly burned for needs such as home heating and cooking food. When a reactant is completely combusted in excess. When C3H8 is burned in oxygen the products are A C H2.
C₃H₈ 5 O₂ --------- 4 H₂O 3 CO₂ Heat The above equation is in the presence of excess oxygen. If 441 g of propane react completely with 1600 g of oxygen 1320 g of carbon dioxide are formed. Write a formula equation for the reaction that shows the physical states of all compounds.
C3H8 O2 CO2 H2O Select one. 80 MOLES OF WATER are produced when 200 moles of propane are burned in excess oxygen on a gas grill. C₃H₈ 10 O₂ 3 CO₂ 4 H₂O.
H 2 O II. B 0525 mol O2. Then 0105 moles of C₂H₈ will require X moles of O₂.
The products of the reaction are carbon dioxide CO2 and water H2O. A mole 602 x 1023 molecules. Combustion occurs when a substance is burned with limited oxygen producing a more diverse range of products than that of complete combustion.
Propane C 3 H 8 is burned in an oxygen atmosphere with 10 deficit oxygen with respect to the stoichiometric requirement. Propane on reaction with Oxygen produces CO₂ and water. 79692 results page 12 ScienceRespiratory and Circulatory Systems What function do the alveoli perform.
When propane gas C3H8 is burned with oxygen the products are carbon dioxide and water. C3H8 g O2 g CO2 g H2O l Assume that liquid water forms in item 5. Heat is produced at 58 kcal mol of water.
Propane gas C3H8 burns completely in the presence of oxygen gas O2 to yield carbon dioxide gas CO2 and water vapor H2O. 1 question If 15606 g of propane C3H8 is burned in excess oxygen how many grams of water are formed. When carbon is burned in air it reacts with oxygen to form carbon dioxide.
CO 2 H 2 O. According to Balance equation 1 mole of C₂H₈ required 10 moles of O₂. C 0875 mol O2.
Solution for when hydrogen and oxygen are burned to produce water. Write a balanced equation for this reaction. Write a formula equation for the reaction that shows the physical states of all compounds.
Propane C_3H_8 a fuel used in many outdoor grills burns in oxygen to produce carbon dioxide gas CO_2 and water vapor H_2O as shown in the reaction below. D 0905 mol O2. When 264 g of carbon were burned in the.
Assume that liquuid water forms in item 5. B CH2 H2O. CO 2 H 2.

When C3h8 Is Burned In Oxygen The Products Are Lisbdnet Com

When C3h8 Is Burned In Oxygen The Products Are Lisbdnet Com

Complete Combustion Of Propane C3h8 Balanced Equation Youtube
0 Comments